Considering their origin, we can distinguish three categories of wastewater:
- Domestic water: These come from various domestic water uses. They mainly carry organic pollution. They are divided into household wastewater, originating from bathrooms and kitchens, generally containing detergents, grease, solvents, organic debris, etc.; and tap water, which consists of water loaded with various nitrogenous organic materials and fecal germs.
- Industrial water: These are very different from domestic wastewater. Their characteristics vary from one industry to another. In addition to organic, nitrogenous, or phosphorus materials, they may also contain toxic products, solvents, heavy metals, organic micropollutants, and hydrocarbons.
- Rainwater: These, too, can cause significant pollution of watercourses, especially during rainy periods. Rainwater collects impurities from contact with the air (industrial fumes) and, as it runs off, picks up residues deposited on roofs and urban roads (drainage oils, fuels, tire residues, and heavy metals...).
- There are several methods used to purify polluted water, depending on the type and extent of contamination. The major six methods used for wastewater purification are given in Figure 4.

These are all practical and easy procedures but do not remove all harmful metal ions from wastewater. In addition, these procedures create solid waste toxic loams and need significant energy, which poses extra environmental issues . All the reported wastewater treatment methods are presented in Figure 5. Physico-chemical processes have many advantages over other processes such as simple and controlled operation, and flexible and temperature controlled. However, these processes have some drawbacks such as high energy requirements and high cost.
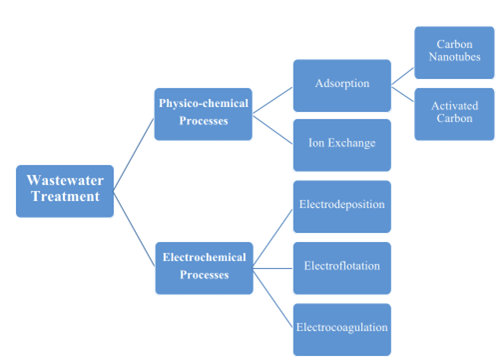
Figure 5: Flow chart of classification of wastewater treatment techniques and methods (Azimi et al. 2017)
V.1. Adsorption
Adsorption is a significant technique used in various treatment processes, such as drinking water purification, industrial wastewater treatment, and remediation of contaminated groundwater or soil. This technique involves the adherence of contaminants to the surface of an adsorbent material (activated carbon, zeolites, certain clays, and specialized resins, etc.)[1] through physical or chemical interactions, effectively removing them from the water. Adsorption process helps remove organic compounds, heavy metals, dyes, and other impurities. In some cases, the adsorbent material can be regenerated by desorbing or removing the adsorbed pollutants, allowing the adsorbent to be reused.
The adsorption effectiveness depends on factors like the specific adsorbent used, contact time, concentration of pollutants, and water chemistry. Although effective, adsorption might not be suitable for treating large volumes of wastewater due to the need for continuous replacement or regeneration of the adsorbent material, which can add to operational costs.
V.2. Magnetically Assisted Processes
Magnetically Assisted Processes represent an innovative approach in wastewater treatment, harnessing magnetic fields to enhance purification methods. The primary application involves the use of magnetic fields to separate contaminants or particles from water. This is particularly effective for removing magnetic or paramagnetic particles from the wastewater. The use of magnetic nanoparticles or magnetic materials in conjunction with the application of a magnetic field facilitates the separation of contaminants. Nanoparticles can be functionalized to attract specific pollutants, aiding in their removal. Magnetic fields can enhance traditional filtration processes by enabling the capture and removal of particles that might otherwise pass through standard filters. In some cases, specific contaminants are tagged or treated with magnetic agents, allowing them to be selectively removed through magnetic processes.
Magnetically assisted processes find applications in various industries and laboratories for wastewater treatment, especially in scenarios where traditional methods might be inadequate. These processes offer potential for improved efficiency and scalability in wastewater treatment, although their widespread adoption might still be in the development phase due to specific equipment requirements and operational complexities.
V.3. Biotechnology:
Biotechnology plays a significant role in wastewater purification, leveraging biological processes or organisms to treat and purify wastewater.
- Microbial Activity: utilizes microorganisms like bacteria, fungi, algae, or protozoa to break down or consume contaminants in wastewater. These organisms can metabolize organic matter, nutrients, and pollutants.
- Bioremediation: involves the use of microorganisms to degrade or neutralize pollutants present in the water. It's particularly effective in treating organic pollutants, hydrocarbons, and certain heavy metals.
- Activated Sludge Process: a common biotechnological method that uses a combination of microorganisms to treat sewage and industrial wastewater. This process involves aeration and the growth of microbial communities that consume organic matter.
- Constructed Wetlands: mimic natural wetland ecosystems by using plants, soil, and microorganisms to treat wastewater. The vegetation and microbial communities in these systems help filter and remove pollutants.
- Anaerobic Digestion: utilizes bacteria in an oxygen-deprived environment to break down organic matter, producing biogas (mostly methane) that can be used as an energy source.
- Genetically Engineered Organisms: research is ongoing in the development of genetically modified microorganisms that can more efficiently break down specific contaminants.
- Biofilters and Biosorption: implement biological materials or living organisms to filter or adsorb pollutants from wastewater. This includes using materials like activated carbon or certain plants to absorb contaminants.
Biotechnological purification methods are often environmentally friendly, cost-effective, and can handle a wide range of pollutants. Despite their efficacy, they may have limitations in treating certain types of contaminants or require longer treatment times compared to some chemical-based processes.
V.4. Ionizing Radiation Processes:
Ionizing radiation processes represent a specialized technique in wastewater treatment, utilizing radiation to treat and disinfect water.
Types of Ionizing Radiation: Techniques may involve gamma radiation, electron beam irradiation, or ultraviolet (UV) radiation. Each type of radiation has specific applications and effectiveness in treating contaminants.
- Microbial Inactivation: Ionizing radiation disrupts the DNA or cellular structures of microorganisms, rendering them unable to multiply or causing their death. This method effectively sterilizes or disinfects water by destroying bacteria, viruses, and other pathogens.
- Chemical Decomposition: The radiation breaks down organic compounds and pollutants present in the water. It can also oxidize certain chemicals, aiding in their removal.
- Disinfection of Water: Particularly effective in disinfecting drinking water or wastewater intended for reuse, such as in agriculture or industrial processes.
- Process and Dosage Control: Careful control of the radiation dosage and exposure time is essential to ensure effective treatment without adverse effects on the environment or human health.
- Industrial Applications: Ionizing radiation processes are often used in large-scale water treatment facilities, especially in situations where conventional treatment methods might be insufficient or where high levels of disinfection are required.
- Advantages and Challenges: This technique can efficiently eliminate pathogens and some pollutants, but it requires specialized equipment, expertise, and strict safety protocols. Concerns also exist about the generation of by-products or potential changes in water chemistry due to radiation exposure.
- Complementary Treatment: Ionizing radiation processes might be used in combination with other treatment methods, such as filtration or chemical disinfection, to achieve comprehensive water purification.
V.5. Membrane Processes
Membrane processes are a prominent and versatile method used in wastewater purification, employing semi-permeable membranes (typically made of polymers, ceramics, or metals) contain pores or channels of specific sizes that allow selective passage of water and molecules of contaminants based on their size and charge. This method efficiently eliminates various contaminants like suspended solids, pathogens, viruses, bacteria, organic compounds, dissolved ions, and pollutants. The wastewater purification by membrane processes includes several techniques such as:
- Reverse Osmosis (RO): uses pressure to force water through a semi-permeable membrane, removing dissolved salts, organic compounds, and other contaminants.
- Ultrafiltration (UF): removes larger particles, colloids, and macromolecules.
- Nanofiltration (NF): operates at a level between RO and UF, removing smaller ions and organic matter.
- Microfiltration (MF): eliminates suspended solids, bacteria, and some larger particles.
Membrane processes (widely used in municipal wastewater treatment, industrial processes, drinking water production, desalination and treatment of wastewater for reuse) offer high efficiency, compact system design and versatility in the treatment of different types of contaminants. They can achieve high quality water standards.
The most important challenges for this purification method are: (1) membrane fouling (accumulation of particles or contaminants on the membrane surface, (2) high operational costs (energy consumption and membrane replacement) and (3) need for pretreatment to reduce fouling are significant challenges. Ongoing research focuses on improving membrane materials, developing antifouling techniques, improving energy efficiency and reducing operating costs.
V.6. Catalytic Processes
Catalytic processes are significant in wastewater purification, involving the use of catalysts[2] (metals, metal oxides, or specific compounds) to enhance the breakdown or transformation of contaminants in water. Catalytic processes are used in various treatment techniques such as the removal of organic pollutants, degradation of dyes, elimination of pharmaceutical residues, and reduction of toxins. They typically involve oxidation (where contaminants are oxidized) or reduction (where contaminants are reduced) reactions, breaking down pollutants into less harmful substances. The advanced Oxidation Processes (AOPs) use catalysts along with oxidizing agents (like ozone, hydrogen peroxide, or UV light) to generate highly reactive radicals that efficiently degrade organic pollutants. Catalytic processes in water treatment often involve heterogeneous catalysis, where the catalyst is in a different phase from the reactants (for example, a solid catalyst in a liquid phase). Photocatalysis: Involves using catalysts (often semiconductors like titanium dioxide) activated by light (UV or visible) to accelerate chemical reactions that degrade contaminants.
Catalytic processes offer many advantages such as high efficiency in breaking down pollutants, selectivity towards specific contaminants and the ability to work in milder conditions compared to some traditional methods. Challenges to this method include the need for specific catalysts for different pollutants, potential catalyst deactivation due to fouling or poisoning, and cost considerations associated with catalysts and energy consumption.
[1] These materials have a high surface area, allowing them to attract and retain contaminants effectively.
[2] Catalysts are substances that facilitate chemical reactions without being consumed themselves.